For US Healthcare Professionals
In the trial, each patient was treated with both VYJUVEK and placebo1
Tap the image to enlarge.
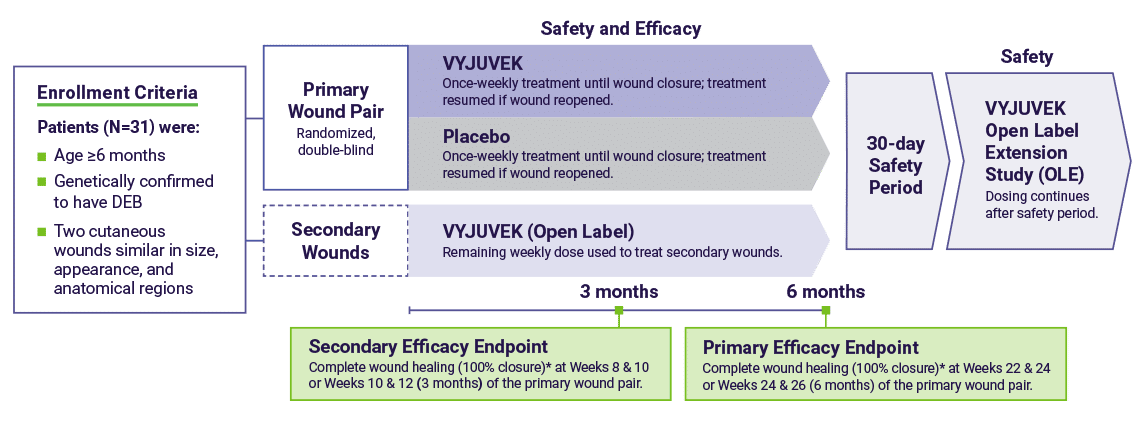
Complete (100%) wound closure was defined as durable wound closure (skin re-epithelialization without drainage) evaluated at two consecutive visits two weeks apart.2
| Patient demographics/characteristics | Total patients (N=31) |
|---|---|
| Age, years | |
| Mean (SD) | 17.2 (10.7) |
| Range | 1-44 |
| Age category, n (%) | |
| ≤12 years | 10 (32.3) |
| >12 to ≤18 years | 9 (29.0) |
| >18 years | 12 (38.7) |
| Sex, n (%) | |
| Male | 20 (64.5) |
| Female | 11 (35.5) |
| Race, n (%) | |
| White | 20 (64.5) |
| Asian | 6 (19.4) |
| American Indian or Alaska Native | 5 (16.1) |
| Genotype, n (%) | |
| Dominant DEB (DDEB) | 1 (3.2) |
| Recessive DEB (RDEB) | 30 (96.8) |
Inclusion Criteria
Exclusion Criteria
VYJUVEK is a topical gel indicated for the treatment of wounds in patients 6 months of age and older with dystrophic epidermolysis bullosa (DEB) with mutation(s) in the collagen type VII (COL7A1) gene.
WARNINGS AND PRECAUTIONS
Accidental Exposure to VYJUVEK gel: VYJUVEK will not replicate in the subject’s cells and does not integrate into the subject cells’ native genetic material. For precautions, avoid direct contact with treated wounds (e.g., touching or scratching) and dressings of treated wounds for approximately 24 hours following treatment. Wear protective gloves when assisting subjects with changing wound dressings and handling the disposal. In the event of accidental exposure (e.g., through a splash to the eyes or mucous membranes), flush with clean water for at least 15 minutes.
Clean all surfaces that may have come in contact with VYJUVEK biological suspension or gel and treat all spills with a virucidal agent.
Dispose all materials (e.g., vial, syringe, needle, cleaning materials) that may have come in contact with VYJUVEK biological suspension or gel into a biohazard bag or container.
ADVERSE REACTIONSThe most common adverse reactions (>5%) were itching, chills, redness, rash, cough, and runny nose.
1. VYJUVEK [prescribing information]. Krystal Biotech, Inc.; 2023.
2. Marinkovich MP, Gonzalez ME, Guide S, et al. GEM-3: a phase 3 study of beremagene geperpavec (B-VEC), an investigational, topical gene therapy, for the treatment of dystrophic epidermolysis bullosa (DEB). Presented at 2022 AAD Annual Meeting; March 27, 2022; Boston, MA.
3. Krystal Biotech, Inc. A phase III double blinded, placebo-controlled, efficacy and safety study of beremagene geperpavec (B-VEC, previously “KB103”) for the treatment of dystrophic epidermolyis bullosa (DEB). ClinicalTrials.gov identifier: NCT04491604. Updated August 3, 2022. Accessed January 10, 2023. https://clinicaltrials.gov/ct2/show/record/NCT04491604
VYJUVEK is a topical gel indicated for the treatment of wounds in patients 6 months of age and older with dystrophic epidermolysis bullosa (DEB) with mutation(s) in the collagen type VII (COL7A1) gene.
WARNINGS AND PRECAUTIONS
Accidental Exposure to VYJUVEK gel: VYJUVEK will not replicate in the subject’s cells and does not integrate into the subject cells’ native genetic material. For precautions, avoid direct contact with treated wounds (e.g., touching or scratching) and dressings of treated wounds for approximately 24 hours following treatment. Wear protective gloves when assisting subjects with changing wound dressings and handling the disposal. In the event of accidental exposure (e.g., through a splash to the eyes or mucous membranes), flush with clean water for at least 15 minutes.
Clean all surfaces that may have come in contact with VYJUVEK biological suspension or gel and treat all spills with a virucidal agent.
Dispose all materials (e.g., vial, syringe, needle, cleaning materials) that may have come in contact with VYJUVEK biological suspension or gel into a biohazard bag or container.
ADVERSE REACTIONSThe most common adverse reactions (>5%) were itching, chills, redness, rash, cough, and runny nose.
1. VYJUVEK [prescribing information]. Krystal Biotech, Inc.; 2023.
2. Marinkovich MP, Gonzalez ME, Guide S, et al. GEM-3: a phase 3 study of beremagene geperpavec (B-VEC), an investigational, topical gene therapy, for the treatment of dystrophic epidermolysis bullosa (DEB). Presented at 2022 AAD Annual Meeting; March 27, 2022; Boston, MA.
3. Krystal Biotech, Inc. A phase III double blinded, placebo-controlled, efficacy and safety study of beremagene geperpavec (B-VEC, previously “KB103”) for the treatment of dystrophic epidermolyis bullosa (DEB). ClinicalTrials.gov identifier: NCT04491604. Updated August 3, 2022. Accessed January 10, 2023. https://clinicaltrials.gov/ct2/show/record/NCT04491604
You are about to leave the VYJUVEKHCP.com website
Are you sure you want to leave?