About VYJUVEK
VYJUVEK is the FIRST AND ONLY treatment that addresses the GENETIC CAUSE OF DEB to provide POWERFUL WOUND HEALING in a topical gel1
VYJUVEK is indicated for the treatment of wounds in patients 6 months and older with dystrophic epidermolysis bullosa (DEB) with the COL7A1 gene mutation.1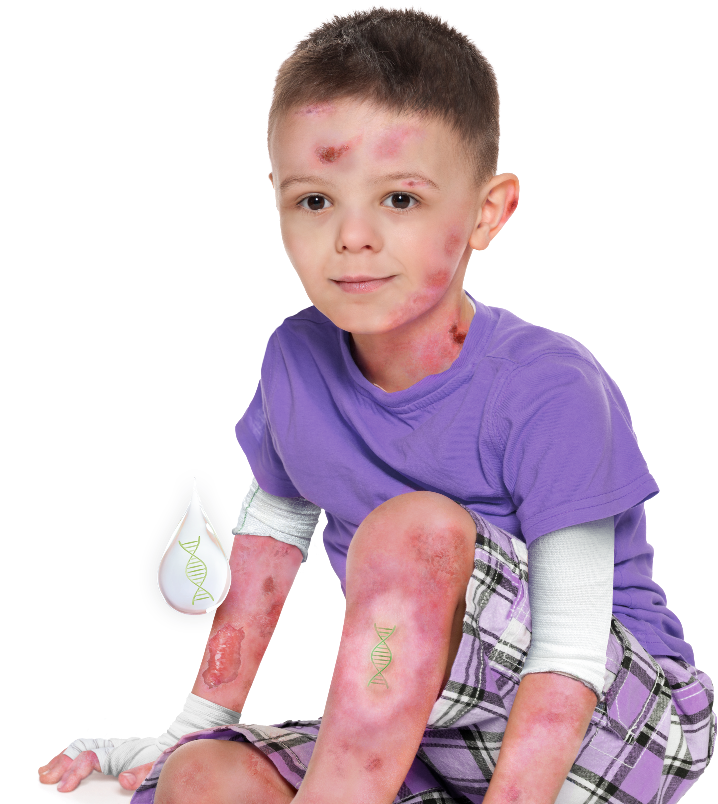
Mechanism of Action
Topical COL7A1 gene delivery
- In DEB, the mutated COL7A1 gene leads to reduced or absent collagen VII, causing insufficient formation of anchoring fibrils and resulting in fragile skin1
- VYJUVEK delivers 2 functional copies of the COL7A1 gene directly into keratinocyte and fibroblast cells of open wounds2
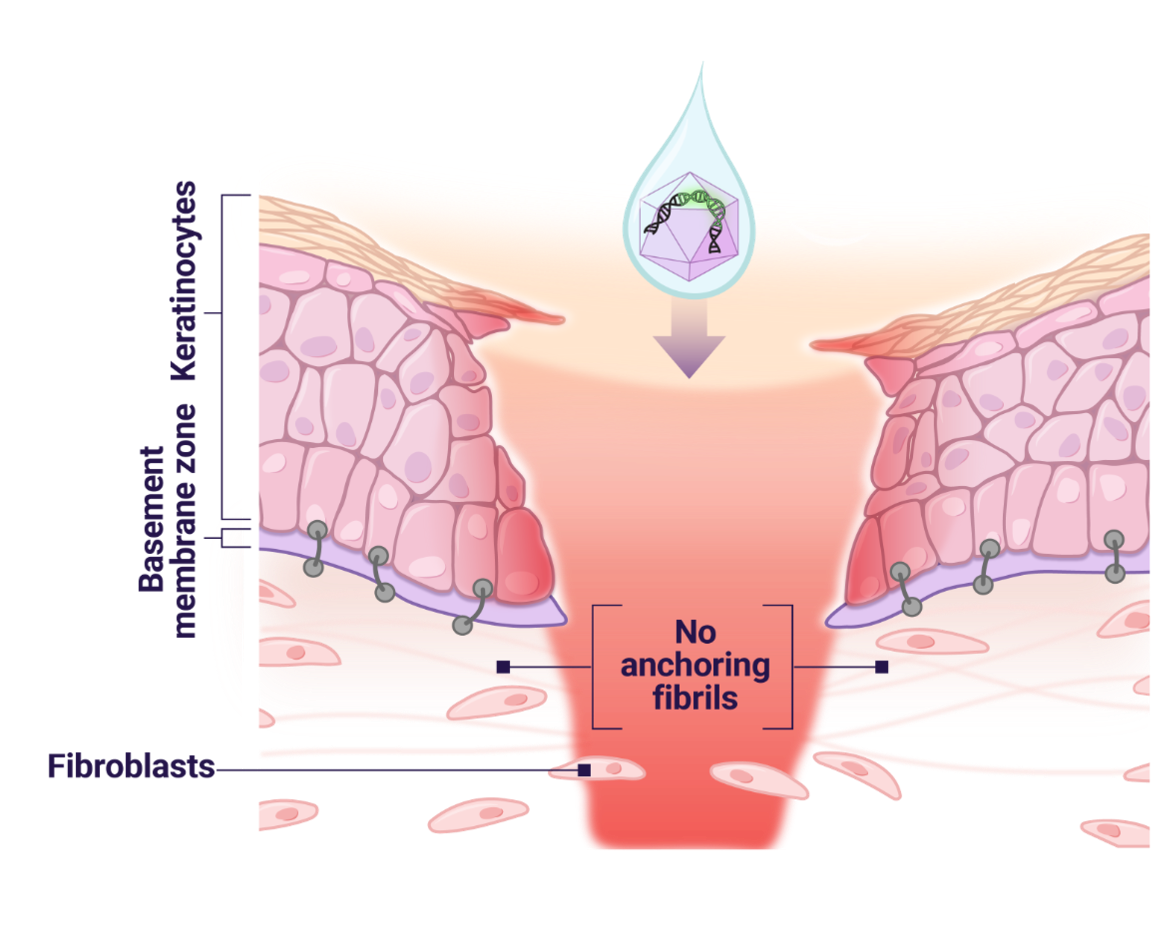

Anchoring fibril production
- The functional genes allow the body to produce type VII collagen protein, which is used to create and assemble anchoring fibrils1
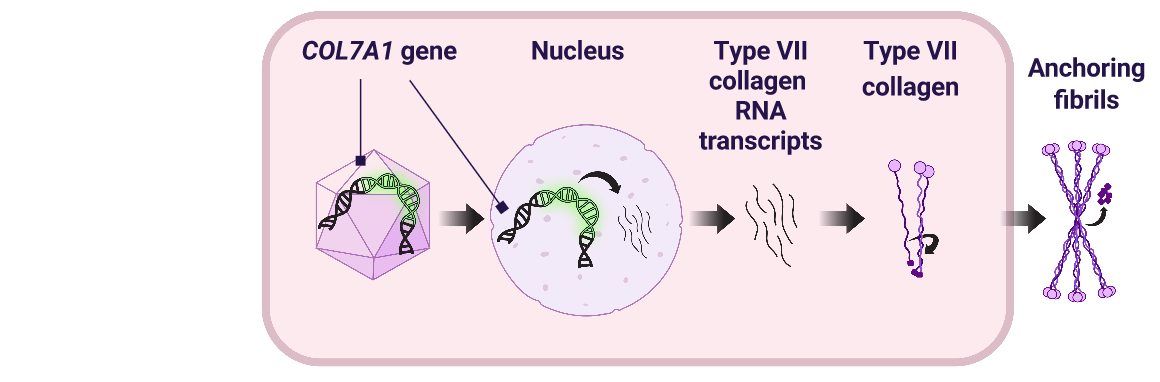

Promotion of wound healing
- Anchoring fibrils help bind the epidermis and dermis together to promote wound closure1

See how VYJUVEK works
See how VYJUVEK was studied
Study DesignVYJUVEK is a topical gel indicated for the treatment of wounds in patients 6 months of age and older with dystrophic epidermolysis bullosa (DEB) with mutation(s) in the collagen type VII (COL7A1) gene.
WARNINGS AND PRECAUTIONS
Accidental Exposure to VYJUVEK gel: VYJUVEK will not replicate in the subject’s cells and does not integrate into the subject cells’ native genetic material. For precautions, avoid direct contact with treated wounds (e.g., touching or scratching) and dressings of treated wounds for approximately 24 hours following treatment. Wear protective gloves when assisting subjects with changing wound dressings and handling the disposal. In the event of accidental exposure (e.g., through a splash to the eyes or mucous membranes), flush with clean water for at least 15 minutes.
Clean all surfaces that may have come in contact with VYJUVEK biological suspension or gel and treat all spills with a virucidal agent.
Dispose all materials (e.g., vial, syringe, needle, cleaning materials) that may have come in contact with VYJUVEK biological suspension or gel into a biohazard bag or container.
ADVERSE REACTIONSThe most common adverse reactions (>5%) were itching, chills, redness, rash, cough, and runny nose.
- Please see Important Safety Information above and click here for full Prescribing Information.
1. VYJUVEK [prescribing information]. Krystal Biotech, Inc.; 2023.
2. Gurevich I, Agarwal P, Zhang P, et al. In vivo topical gene therapy for recessive dystrophic epidermolysis bullosa: a phase 1 and 2 trial. Nat Med. 2022. doi:10.1038/s41591-022-01737-y
VYJUVEK is a topical gel indicated for the treatment of wounds in patients 6 months of age and older with dystrophic epidermolysis bullosa (DEB) with mutation(s) in the collagen type VII (COL7A1) gene.
WARNINGS AND PRECAUTIONS
Accidental Exposure to VYJUVEK gel: VYJUVEK will not replicate in the subject’s cells and does not integrate into the subject cells’ native genetic material. For precautions, avoid direct contact with treated wounds (e.g., touching or scratching) and dressings of treated wounds for approximately 24 hours following treatment. Wear protective gloves when assisting subjects with changing wound dressings and handling the disposal. In the event of accidental exposure (e.g., through a splash to the eyes or mucous membranes), flush with clean water for at least 15 minutes.
Clean all surfaces that may have come in contact with VYJUVEK biological suspension or gel and treat all spills with a virucidal agent.
Dispose all materials (e.g., vial, syringe, needle, cleaning materials) that may have come in contact with VYJUVEK biological suspension or gel into a biohazard bag or container.
ADVERSE REACTIONSThe most common adverse reactions (>5%) were itching, chills, redness, rash, cough, and runny nose.
- Please see Important Safety Information above and click here for full Prescribing Information.
1. VYJUVEK [prescribing information]. Krystal Biotech, Inc.; 2023.
2. Gurevich I, Agarwal P, Zhang P, et al. In vivo topical gene therapy for recessive dystrophic epidermolysis bullosa: a phase 1 and 2 trial. Nat Med. 2022. doi:10.1038/s41591-022-01737-y
You are about to leave the VYJUVEKHCP.com website
Are you sure you want to leave?
